Rapid an Accurate Detection of Whooping Cough in Clinical Samples with the Vivalytic Bordetella
Background
Despite a high vaccination coverage in the EU, whooping cough is not only present in Europe, but is dramatically on the rise in the post-pandemic era. Several countries reported outbreaks or increasing numbers in 2023/241,2:
- Denmark: Infection cases 4-fold higher in 2023 (compared to 2022)
- Spain: Infection cases 7-fold higher in 2023 (compared to 2022)
- United Kingdom: 553 cases in January 2024 (2023 858 per annum; 7-fold)
- Croatia: 6.261 cases reported in the first 3 months in 2024
Methods
411 nasopharyngeal specimens (all in Copan eNAT™ swabs) collected from symptomatic patients in Croatia were tested in our lab.
All 411 samples were pre-characterized with the reference test.
In addition, we received 11 positive samples from a hospital in Split (Croatia).
To accurately validate the new Vivalytic Bordetella assay (Bosch Healthcare Solutions, Waiblingen, Germany) we used 150 spiked samples:
50 samples of each virulent species Bordetella pertussis (Bpt), B. parapertussis (Bpa) and B. holmesii (Bho) were spiked with 3 different inocula, low, medium and high (20/50/200 10e3 copies/mL). All 561 samples were compared to the Bordetella Speciation Plus Toxin-OSR (BioGX, Amsterdam/NL) as reference test, which also covers all three virulent Bordetella species. To achieve comparable results both tests were performed within 72 hours.
Discrepant results were tested with a third party test: RIDA Gene Bordetella.
Results
In the 411 Croatian samples we detected 10 Bordetella positive specimens with Vivalytic (2,4%) and 8 with BioGX assay (1,9%).
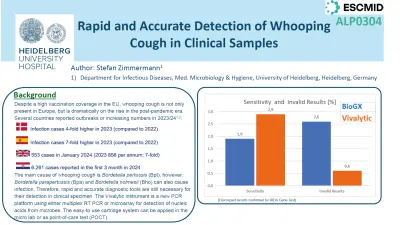
The Vivalytic Bordetella cartridge showed a very high concordance to the reference test 97.7% The positive percent agreement (PPA) was 97.9%. As the positivity rate of the specimen set was rather low, we received additional positive samples (n=11) from a hospital in Split (Croatia). All swabs gave positive results for Bordetella pertussis in the Vivalytic, 100% PPA. Within the 150 spiked samples the PPA was >98% for all tests and >95% for the challenging low inocula samples. Time-to result was only 45min for the Vivalytic test compared to 150min for the comparator assay.
The rate of invalid results of the Vivalytic assay was very low 0,6%.
Conclusion
The Vivalytic Bordetella cartridge demonstrated excellent concordance with a sensitive reference test and delivered accurate and rapid results. The assay is suitable for hospital labs and for outpatients’ settings due to its easy use and short time-to report.
Easy-to use PCR systems with rapid results are suitable also for smaller hospitals and outpatient department. Early identification of infected patients will help to intercept transmission of the current re-emerging whooping cough epidemics.
1ECDC Weekly Communicable Disease Threats Report, Week 51/2023
2Smout E., etal.; BMJ 2024: 385:q736, epub 02 April 2024
