Evaluation of the Vivalytic one Analyser for detecting uropathogenic bacteria and antimicrobial resistances in urine samples of urological patients
Background
The Vivalytic Urinary tract infection (UTI) test, currently under development, represents a qualitative PCR-based microarray test able to detect twenty-one specific uropathogenic bacteria and seven associated antimicrobial resistance genes in a point-of-care (POC) setting in four steps within 146 minutes.
Methods
In September 2023, we performed the Vivalytic UTI test on 126 consecutive urine samples of urological patients in the University Hospital of Giessen, Germany.
We preselected the urine samples for bacteriuria by screening with urine flow cytometry (UFC).
➛ UFC cut-off ≥70 bacteria/μl.
We performed the Vivalytic UTI POC test twice:
(1) Urological department; before transport
(2) Microbiological laboratory; after transport. Results were compared to standard urine culture and antibiotic sensitivity testing according to EUCAST; after transport of urine specimen.
Results
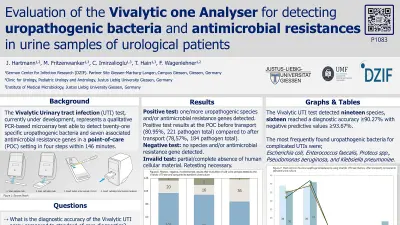
Positive test: one/more uropathogenic species and/or antimicrobial resistance genes detected.
Positive test results at the POC before transport (80.95%, 221 pathogen total) compared to after transport (78,57%, 184 pathogen total).
Negative test: no species and/or antimicrobial resistance gene detected.
Invalid test: partial/complete absence of human cellular material. Retesting necessary.
Conclusion
In this study, the Vivalytic UTI test displayed high sensitivity and specificity in identifying uropathogenic bacteria and antibiotic resistance markers.
We observed a higher degree of concordant pathogen identification at the POC, before transport (p=0.0336). The transport of urine samples influenced the pathogen detection rate and antibiotic susceptibility testing of the Vivalytic UTI analyser.
